
LITHIUM ION CELLS AND THERMAL RUNAWAY
The Lithium ion Cell is the heart of the modern technology of many items used extensively today. The battery in a mobile phone is a lithium ion cell and that in the Apple iPad also. A typical cell is made up of
- an aluminium terminal on which a substance containing a lithium compound is pasted. This substance can be of various complex types Lithium Nickel Cobalt (LNC) and Lithium Manganese Cobalt (LMC) are two examples that have been used extensively. Lithium Ferrous Phosphate (LFP) is a safer chemistry and is preferred for static battery storage.
- a microporous plastic separator on which is pasted a lithium based organic electrolyte, which is flammable.
- a copper terminal on which graphite is pasted. The separator besides keeping the two electrodes apart (thus preventing a short circuit), also allows Lithium ions to pass freely back and forth during the charging and discharging process.
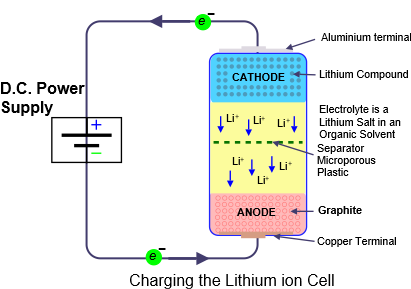
The lithium compounds include iron, nickel and/or cobalt whose electrons are only loosely bound to their central atomic nucleus. Applying a positive voltage to the cathode can move electrons through the outside circuit to the anode while the lithium ions move through the electrolyte from cathode to anode.
In the diagram the lithium ion cell is connected to a DC power supply. As the voltage is applied, positively charged lithium ions move from the cathode through the electrolyte to the graphite anode and nestle between the graphite crystal structure. Meanwhile electrons from the cathode travel twoards the positive teminal of the power supply and then towards the anode. They then meet the lithium ions . The charging process removes electrons from the cathode and unites them with the lithium ions at the anode.
The result is that the cathode has lost electrons which have been deposited at the anode. The cathode is now negatively charged and if the DC power source is replaced by a load such as an electric light bulb or electric motor, the opposite journey takes place. The Lithium ions move back through the electrolyte while the electrons that were at the anode travel through the outside circuit back to the cathode. In doing so the electric current provides useful power.
So it would seem that the lithium ion cell is a providential opportunity to solve all our net zero problems. But unfortunately life is not so simple. These lithium cells are fairly fragile, susceptible to vibration and when fully charged can burst into flame or explode if abused.
Lithium cell abuse
The cells in a Lithium ion battery work well if maintained within specified limits but if abused in any of the following ways, the cell and indeed whole battery could result in a catastrophic event. The types of abuse have been clearly explained by Dr Billy Wu of Imperial College in his youtube presentation on battery fires. The summary is given in the following diagram adapted from his presentation.
Mechanical Abuse occurs if the cell is penetrated by a metal object, such as a nail or a bullet. A short circuit occurs, the temperature rises and thermal runaway can follow.
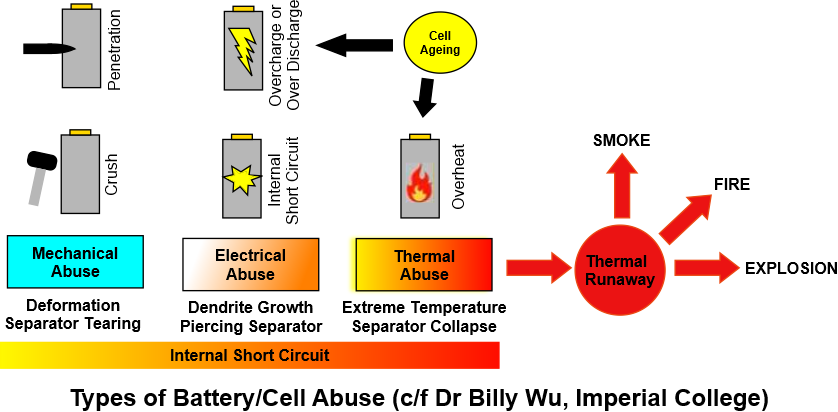
Electrical abuse can occur if the cell is overcharged or discharged to too low a value continuously. This abuse can result in what are called 'dendrites' which whiskers of lithium growing onto the electrodes. If these dendrites get long enough, they can pierce the separator, and cause a short circuit and thermal runaway.
Thermal abuse arises if a cell overheats. Each cell has an internal resistance and during the charge or discharge this will generate heat. The amount of heat generated per cell is small but within each container there would be thousands of cells. The design of a BESS requires the use of air conditioning or other cooling methods to take away this wild heat. If the temperature within the container were to rise, the plastic separator may collapse, a short circuit would follow, and a situation which has been called thermal runaway would ensue.
Thermal Runaway
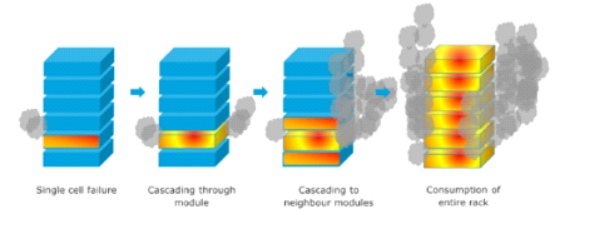
The paper by Dr Edmund Fordham, Professor Wade Allison and Professor Sir David Melville cited earlier describes how lithium battery cells, in certain circumstances, can get overheated and if they reach a certain temperature, they will catch fire and emit noxious gases. If a single cell bursts into flame, it will heat up adjacent cells which will also burst into flame. If the cells are not immediately cooled down a chain reaction will set in and there will be what is called thermal runaway.
- A single cell failing could cascade into neighbouring cells in a module
- Modules could then cascade into neighbouring modules followed by . . .
- the consumption of the entire stack and then the container
A recent paper by Guy Marlair, Amandine Lecocq, Arnaud Bordes, Paul Christensen, and Benjamin Truchot sets out their Key Learnings from Recent Lithium-ion Battery Incidents. They conclude that "No chemistry, not even less reactive LFP lithium-ion chemistry, is exempt from thermal runaway"
They recommend that a fail-safe approach with multi-layer protection barriers should be the future strategy, not just relying on the use of safer materials in the hope of negating the Thermal Runaway hazard.
It follows therefore that any design claiming a fail-safe approach needs to be exhaustively analysed and tested and demonstrated before any decision is made whether or not to give the go ahead.